COAPTIUM® CONNECT is an advanced system designed for sutureless nerve repair. It leverages TISSIUM®’s unique biopolymer platform to create a strong coaptation that eliminates stress at the coaptation site for a tensionless repair.
SIMPLE Design
COAPTIUM® CONNECT allows surgeons to control variability and achieve consistency5.
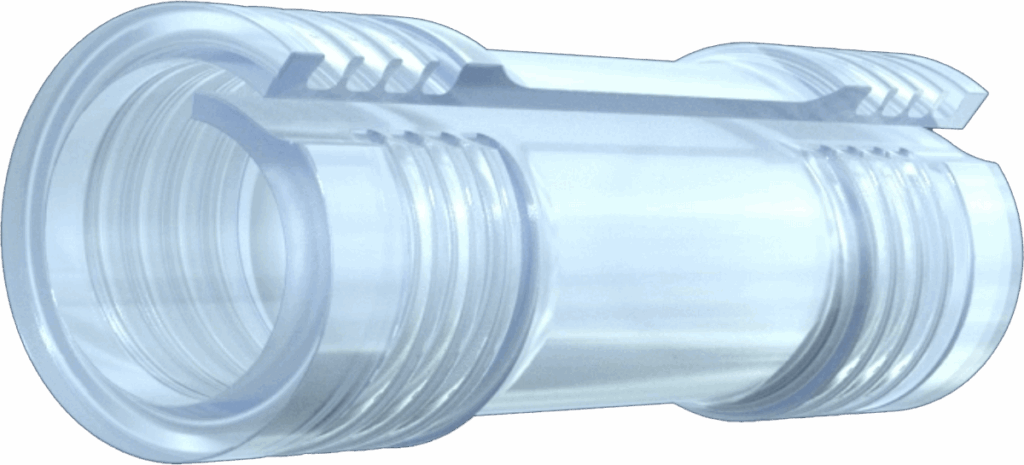
COAPTATION CHAMBER aligns nerve ends and supports healing of the coaptation site
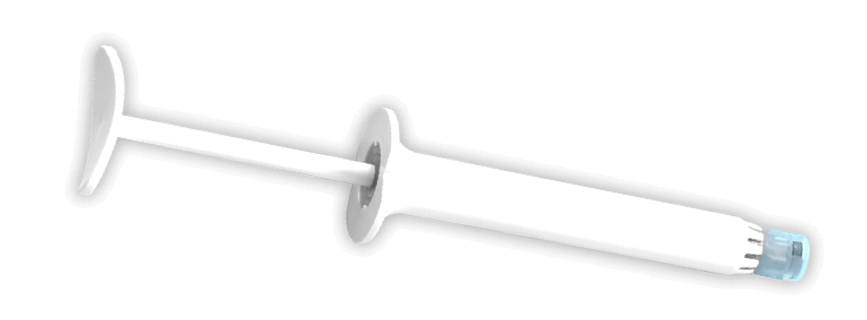
PRE-FILLED SYRINGE WITH COAPTIUM© POLYMER creates a strong and reliable connection
![]()
SILICONE APPLICATOR ensures a controlled connection

TISSIUM® LIGHTactivates viscous pre-polymer into flexible bond
Images not to scale.
See COAPTIUM® CONNECT
in Action
COMPLETE System
COAPTIUM® CONNECT contains everything needed to ensure an optimal repair every time.
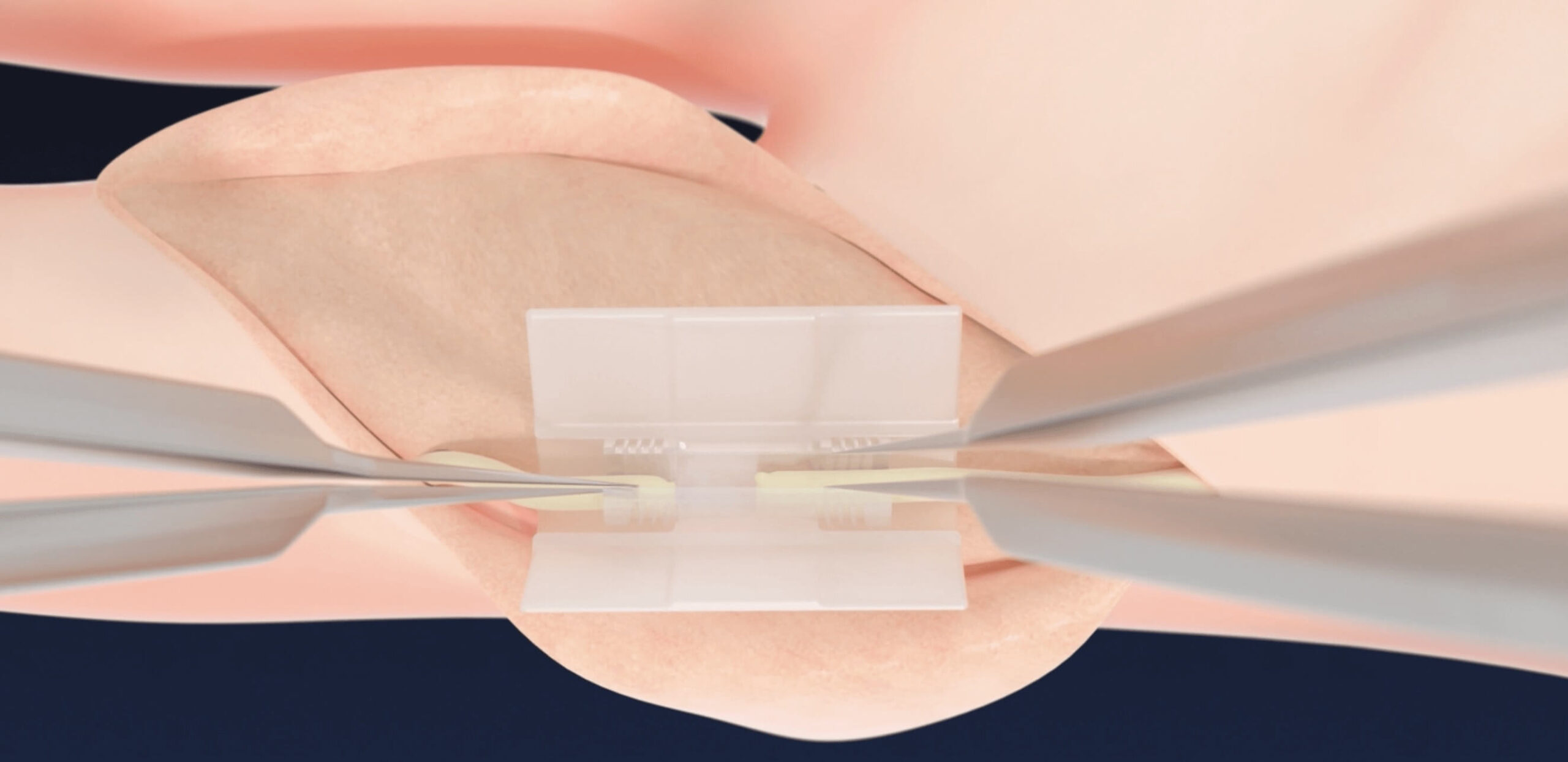
ALIGN Nerve and Insert into Chamber
- Positions nerve ends in their natural axis to maximize fascicle alignment
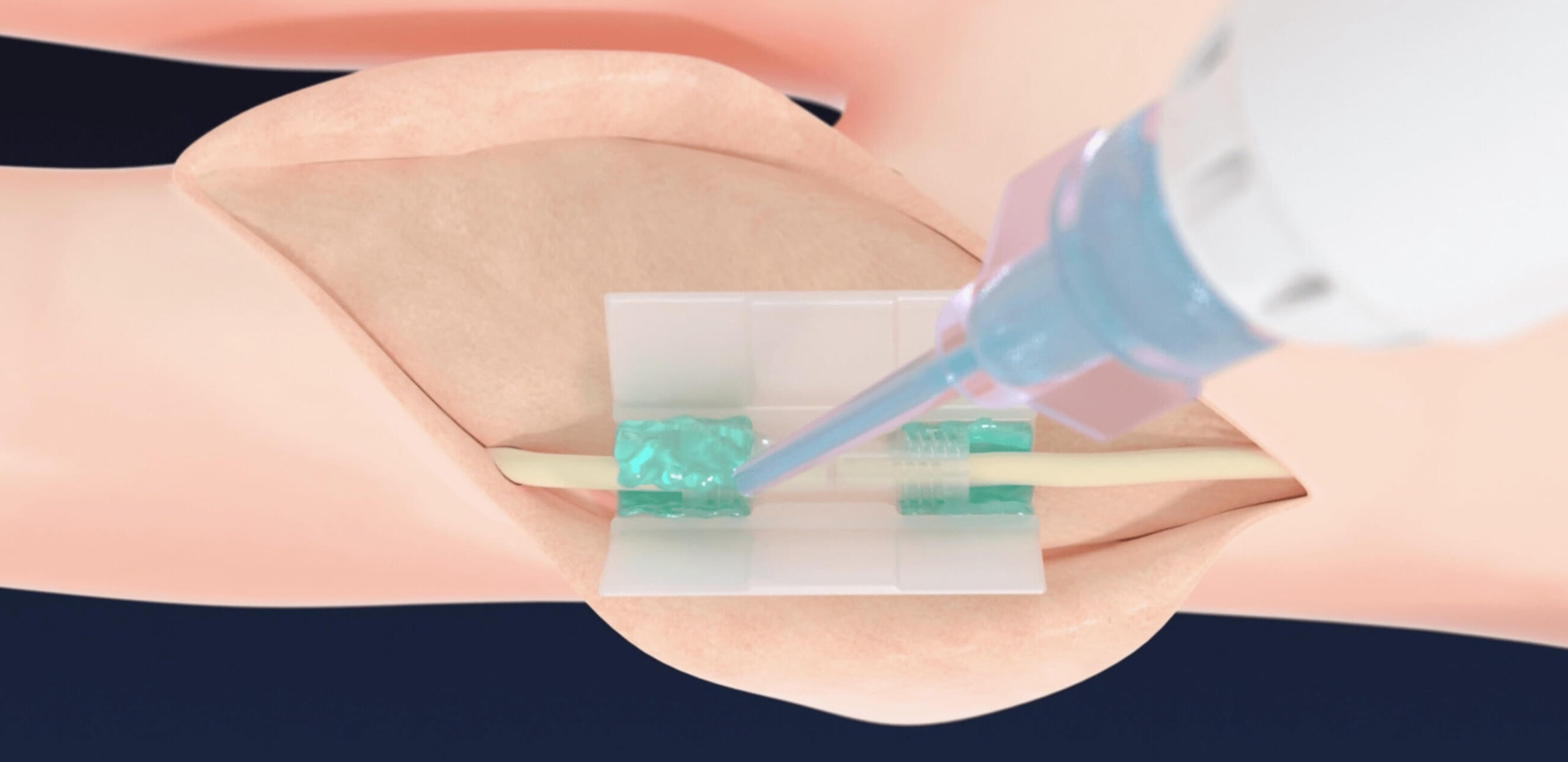
APPLY Polymer
- Secures the free “ends” to the chamber, eliminating any stress at the coaptation site
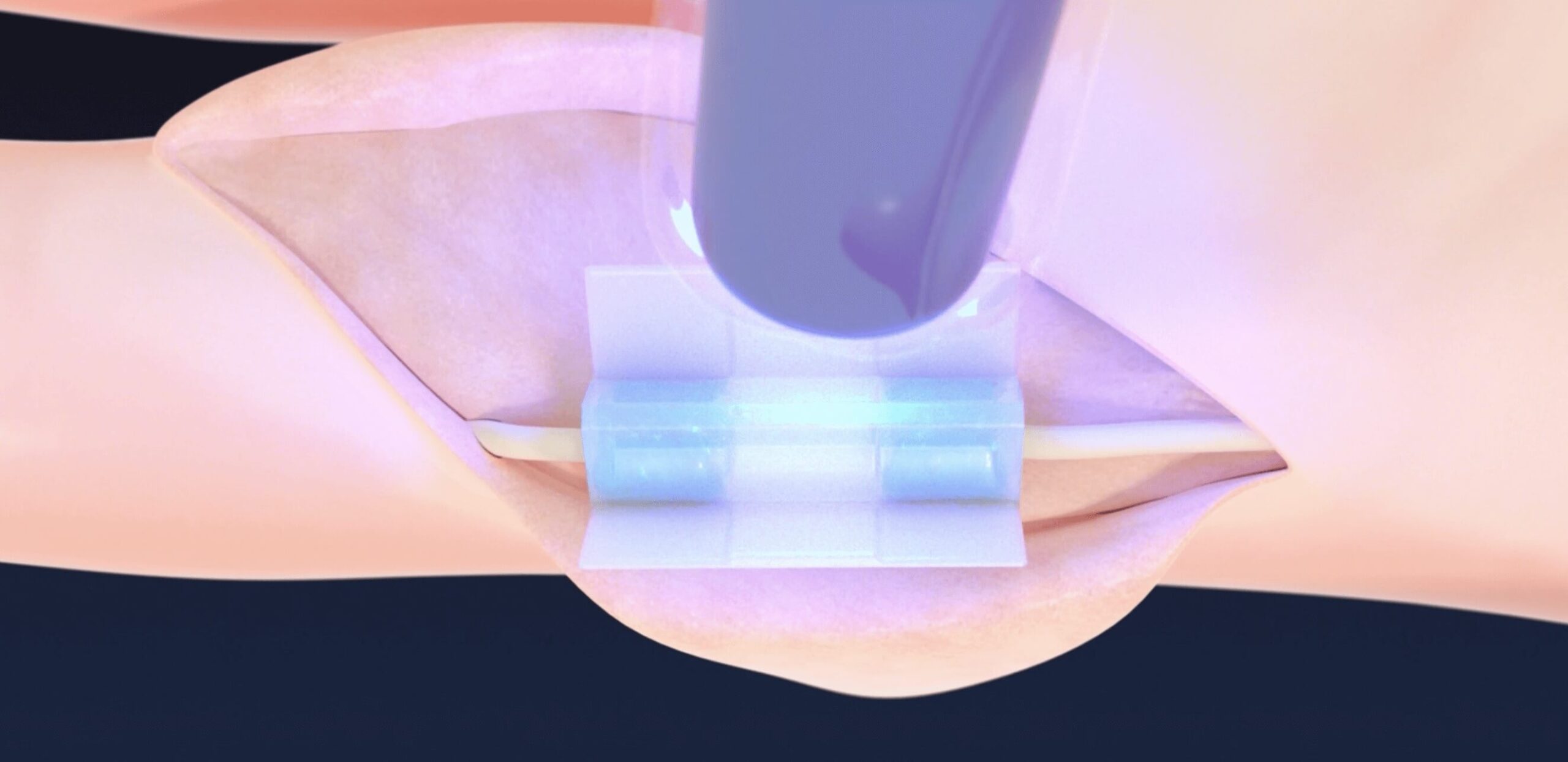
ACTIVATE with TISSIUM® LIGHT
- Delivers precise and controlled light-activation fixation
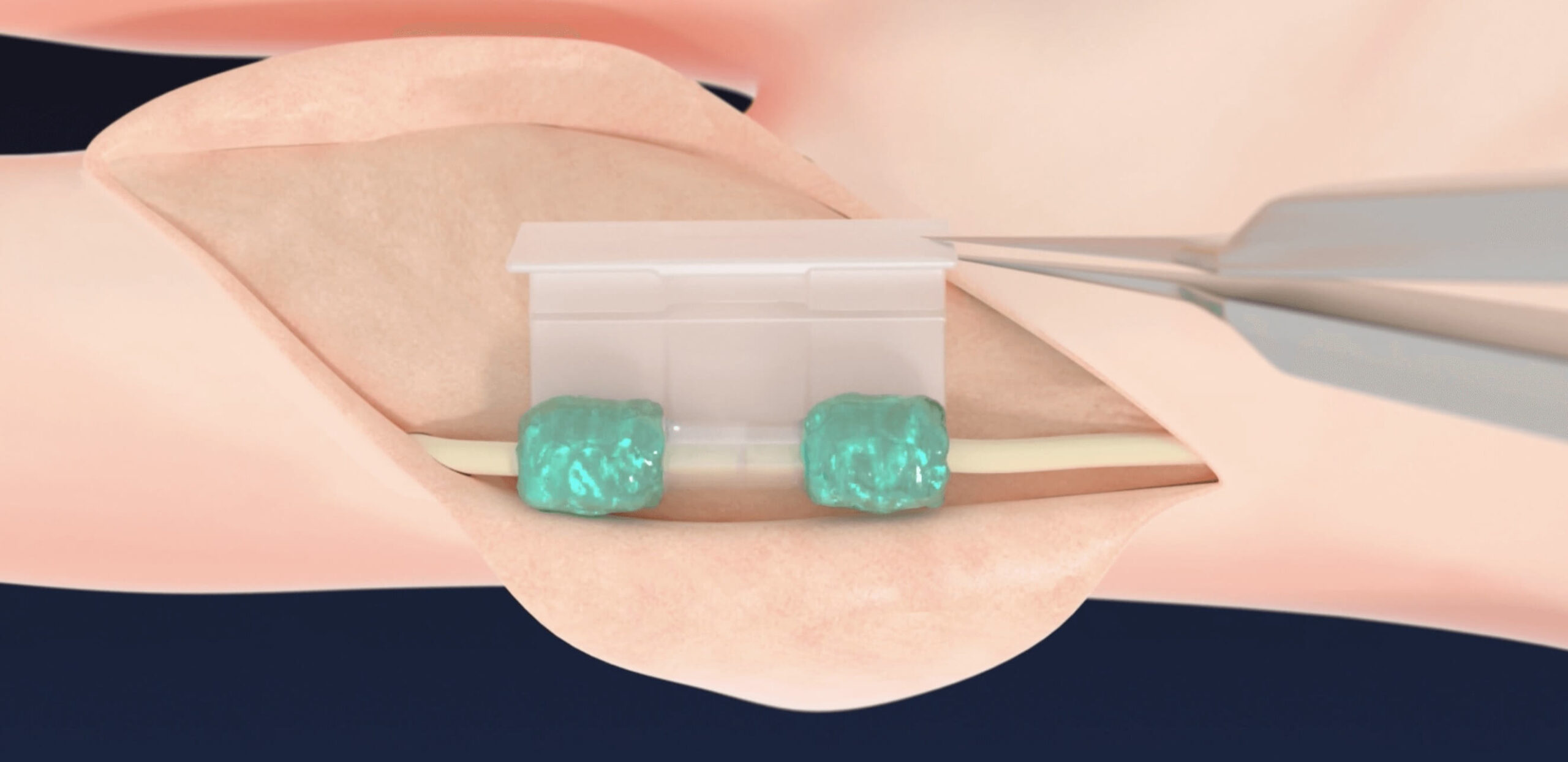
ACHIEVE FUNCTIONAL REPAIR
- Supports nerve healing with our novel biocompatible and bioabsorbable polymer
Images not to scale.
INDICATIONS FOR USE
COAPTIUM® CONNECT with TISSIUM® LIGHT is indicated for the sutureless repair of peripheral nerve injuries not in continuity in which a gap closure ≤ 1 cm is present or can be achieved with flexion of the extremity.
CONTRAINDICATIONS
COAPTIUM® CONNECT is contraindicated in individuals with known or suspected hypersensitivity to aminated polyglycerol sebacate acrylate or the color additive FD&C Blue No. 1 Dye (brilliant blue FCF).
WARNINGS
- DO NOT use this system if the package seal is damaged or open.
- DO NOT use if there is a missing or damaged item. Ensure that all the listed items are present. Check the integrity of all the items in the COAPTIUM® CONNECT system.
- This device is for single use only. DO NOT reuse, re-sterilize, or reprocess as it may lead to unpredictable performance and may lead to its failure and subsequent patient injury.
- DO NOT use past the expiration date.
- DO NOT use the product if the temperature indicator meter reads “> 4.”
- DO NOT cut, trim, or alter the coaptation chamber.
- Keep the COAPTIUM® polymer out of direct light prior to application.
- The aluminum pouch containing the COAPTIUM® polymer is not a sterile barrier and serves as a moisture barrier only.
- The COAPTIUM® Connect with TISSIUM® LIGHT should not be used in patients treated with photosensitizing pharmaceutical products.
PRECAUTIONS
- COAPTIUM® CONNECT has not been evaluated for use in pregnant or nursing women, or in the pediatric population.
- The use of more than 6 COAPTIUM® CONNECT systems per patient per lifetime has not been assessed.
- The safety and effectiveness of the COAPTIUM® CONNECT with TISSIUM® LIGHT has not been evaluated for primary repair of peripheral nerve injuries.
POTENTIAL COMPLICATIONS
- Possible complications can occur with any peripheral nerve repair surgical procedure including pain, infection, decreased or increased nerve sensitivity, and complications associated with use of anesthesia.
REFERENCES
- ASPN 2025 Presentation – Safety and Performance of a Novel Atraumatic Polymer-assisted System for Peripheral Nerve Repair
Compared to Microsutures